Patients may make a number of scheduled visits in a particular study (e.g., a screening visit or a 30-day scheduled visit).
Visits contain specific iCRFs to be completed in that visit; for example during a screening visit a subject may have demographic and vitals information collected.
There are also unscheduled visits that may be completed anytime during a trial, such as in the case of an adverse event.
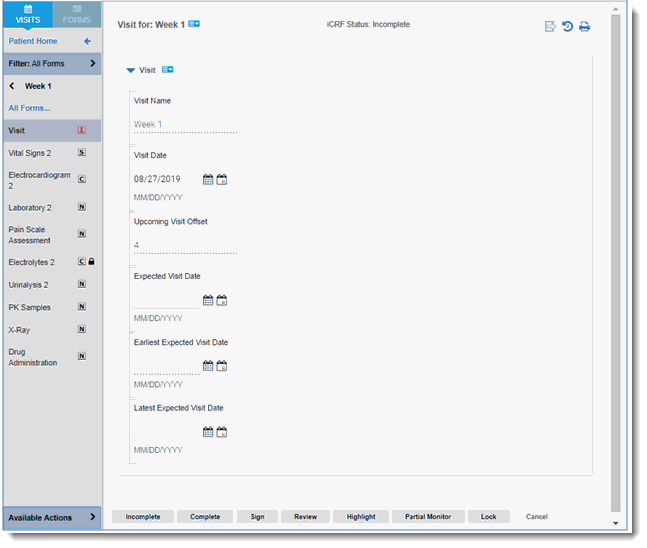
There are no default visits; however, new visits can be created, edited, copied and deleted. Visits are associated with Patients. Below is an example of a visit in TrialMaster.
You can manage visit objects in the following ways:
· Perform bulk operations (copy, delete, or move visits)