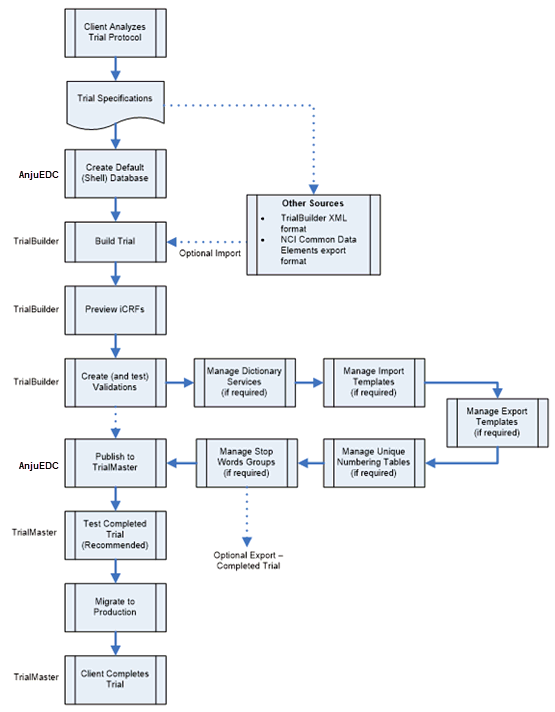
Building a new trial is a consistent process:
1. Client analyzes the trial protocol to create trial specifications.
2. The study builder creates a “shell” database using AnjuEDC. This could also be done by a Data Manager or similar role.
3. The study builder builds the trial by creating the appropriate iCRFs. The trial can also be built using other applications (XML, NCI Common Data Elements, Oracle Clinical tables, etc.) and imported appropriately.
4. The study builder tests the content of the iCRFs. This does not include field validation testing (edits).
5. The study builder creates and tests field validations (edits). The testing is done using the TrialBuilder built-in test feature. This is not a complete and extensive test of the completed trial.
6. The study builder manages the following elements in the trial (if required):
· Dictionary Services
· Import Templates
· Export Templates
· Unique Number Templates
· Stop Word Group(s)
At this point, the study can be exported if required. However, it is strongly recommended to complete thorough testing before attempting to export the trial.
7. The completed trial is thoroughly tested in a separate test environment in TrialMaster (recommended).
8. The completed trial is migrated to a production environment.
9. The client completes the trial using TrialMaster.
See the workflow below to view this process.